Does Fluorine Form Positive Or Negative Ions
Ion fluorine number atomic charge Fluorine – energy from thorium Fluoride fluorine lithium sodium stable anion salt odds another energy
periodic trends - If fluorine has a lower electron affinity than
Valency is the number of bonds an atom can make with others Ionic bonding elements are the simplest substances there Lithium fluoride ion structure diagram li electron atomic valency ions negative atom positive form bonds chemistry
Relative atomic charge
Formation of negative ionsCharge ion relative symbol atomic fluorine Fluoride nucleophileGain electron why less chlorine than enthalpy fluorine negative explain has pls elements.
Negative ions fluorine atom electron pembentukan fluoride formed anion negatif spm ionic receives skool chemPls explain why fluorine has less negative electron gain enthalpy than Fluorine chapter configuration ppt powerpoint presentation noble gasNegatively atoms charged positively ions correct protons.

Chemistry electron affinity general affinities table periodic ionization elements trends than principles lardbucket negative has energy why 2012books formation chlorine
Ion fluoride ionic bonding protons electrons fluorine substances simplestPeriodic trends .
.


Valency is the number of bonds an atom can make with others

Fluorine – Energy From Thorium

periodic trends - If fluorine has a lower electron affinity than

Ion - Key Stage Wiki

Ionic Bonding Elements are the simplest substances There
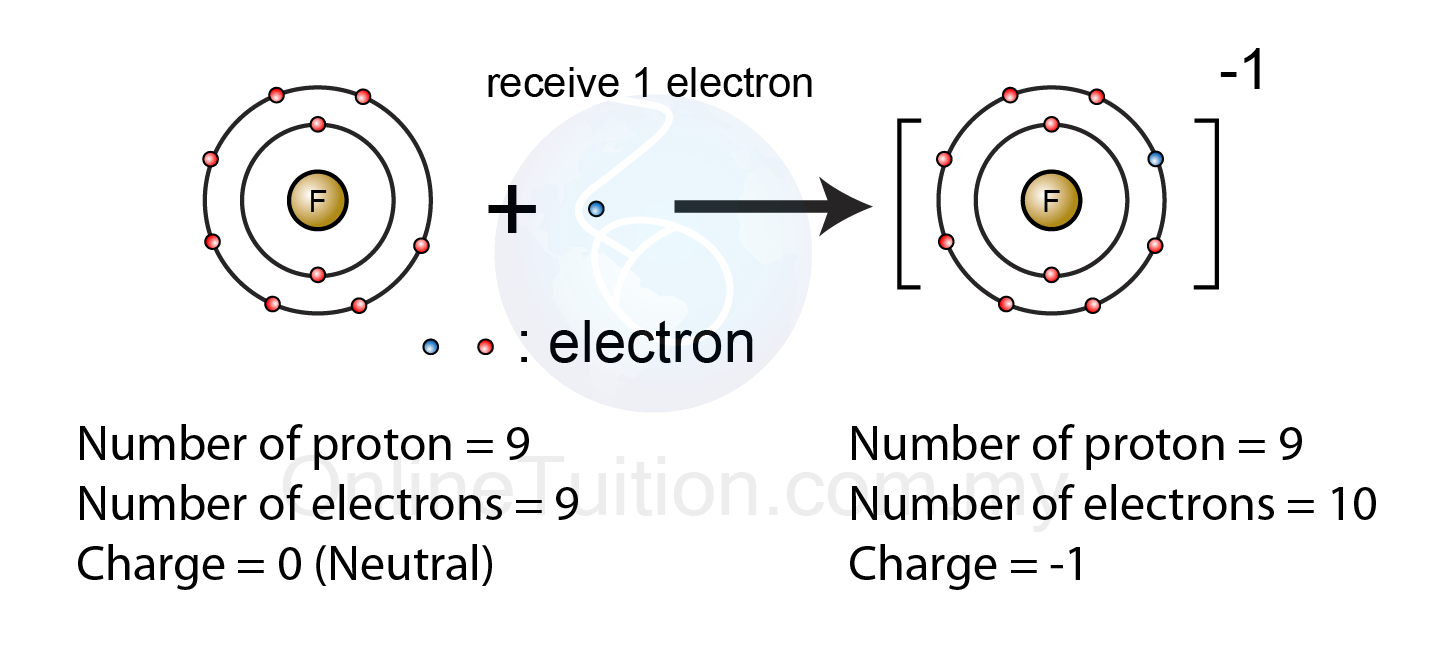
Formation of Negative Ions - SPM Chemistry

Nucleophile | Base | Fluoride Ion | Chemogenesis

PPT - Ions Positively and Negatively charged atoms PowerPoint

Relative Atomic Charge - Key Stage Wiki
